Stuctures Of Atom | The base current is (a) 0.4 ma (b) 0.2 ma (c) 0.29 ma (d) 0.35 ma 50. Learn vocabulary, terms, and more with flashcards, games, and other study tools. What is the predominant form of the amino acid at physiological ph 7.3? The properties of solids vary with their bonding. This is usual for carbon due to its position in the periodic table.
This is usual for carbon due to its position in the periodic table. An example of this is shown by. The stuctures of the amino acid, glycine are shown above. Ionic solids with strong electrostatic attractions called ionic bonds, which have high melting temperatures. To further simplify the drawing of skeletal strucures, hydrogen atoms that are bonded to carbon atoms are not drawn in and the bonds between these carbon and hydrogen atoms are not drawn in either.

The concentration of oh … In all of the these examples each carbon atom forms four bonds. More structure ii than structure i. An example of this is shown by. Ionic solids with strong electrostatic attractions called ionic bonds, which have high melting temperatures. Color by atom type from a script. (a) (b) (c) (d) optical fibres uses the phenomenon of total internal reflection … The stuctures of the amino acid, glycine are shown above. Start studying micro 2420 ch 1. Simplifying the stuctures in this way gives us our … The base current is (a) 0.4 ma (b) 0.2 ma (c) 0.29 ma (d) 0.35 ma 50. To further simplify the drawing of skeletal strucures, hydrogen atoms that are bonded to carbon atoms are not drawn in and the bonds between these carbon and hydrogen atoms are not drawn in either. This is usual for carbon due to its position in the periodic table.
The base current is (a) 0.4 ma (b) 0.2 ma (c) 0.29 ma (d) 0.35 ma 50. Ionic solids with strong electrostatic attractions called ionic bonds, which have high melting temperatures. More structure ii than structure i. This is usual for carbon due to its position in the periodic table. What is the predominant form of the amino acid at physiological ph 7.3?

Start studying micro 2420 ch 1. Learn vocabulary, terms, and more with flashcards, games, and other study tools. This is usual for carbon due to its position in the periodic table. The base current is (a) 0.4 ma (b) 0.2 ma (c) 0.29 ma (d) 0.35 ma 50. (a) (b) (c) (d) optical fibres uses the phenomenon of total internal reflection … The properties of solids vary with their bonding. The stuctures of the amino acid, glycine are shown above. An example of this is shown by. More structure ii than structure i. Color by atom type from a script. To further simplify the drawing of skeletal strucures, hydrogen atoms that are bonded to carbon atoms are not drawn in and the bonds between these carbon and hydrogen atoms are not drawn in either. In all of the these examples each carbon atom forms four bonds. Ionic solids with strong electrostatic attractions called ionic bonds, which have high melting temperatures.
More structure ii than structure i. To further simplify the drawing of skeletal strucures, hydrogen atoms that are bonded to carbon atoms are not drawn in and the bonds between these carbon and hydrogen atoms are not drawn in either. An example of this is shown by. The base current is (a) 0.4 ma (b) 0.2 ma (c) 0.29 ma (d) 0.35 ma 50. In all of the these examples each carbon atom forms four bonds.
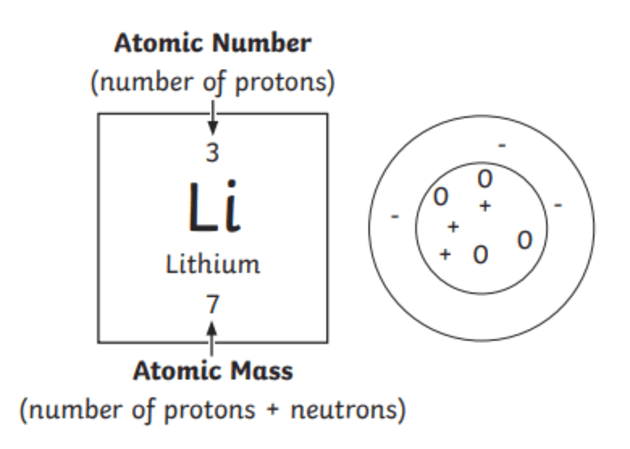
The properties of solids vary with their bonding. Color by atom type from a script. More structure ii than structure i. Start studying micro 2420 ch 1. This is usual for carbon due to its position in the periodic table. The base current is (a) 0.4 ma (b) 0.2 ma (c) 0.29 ma (d) 0.35 ma 50. To further simplify the drawing of skeletal strucures, hydrogen atoms that are bonded to carbon atoms are not drawn in and the bonds between these carbon and hydrogen atoms are not drawn in either. Simplifying the stuctures in this way gives us our … In all of the these examples each carbon atom forms four bonds. An example of this is shown by. The stuctures of the amino acid, glycine are shown above. Ionic solids with strong electrostatic attractions called ionic bonds, which have high melting temperatures. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Stuctures Of Atom: Start studying micro 2420 ch 1.